Best practice for Immunoglobulin Assessment Panels (IAPs)
A number of best practice recommendations have been identified from the IAP survey, the annual MDSAS event and discussion with the immunoglobulin project working group. Work is underway to define the most effective models/s for IAPs and these recommendation s are likely to evolve over time. Whilst not mandatory implementation of these recommendations is expected to contribute to the effective working of an IAP and support the safe and effective use of immunoglobulin:
• Ensure IAP in place
• Terms of Reference (ToR) agreed and reviewed
• ToR to include purpose, membership, frequency, quoracy, accountability and functions
• Independent chair, i.e. not a haematologist, immunologist or neurologist
• Panel membership to include haematology, immunology and neurology representation; minimum of two of these for quoracy
• Consider commissioner engagement (this may be more achievable in cross-trust IAPs)
• Ensure medical director support for the panel and process
• Consider mix of meetings to deliver functions, such as quarterly face to face meetings with virtual meetings for considering urgent requests; process for requests out of hours
• Review of dosing in long-term use of immunoglobulin
• Be prepared to challenge requests, dosing, on-going use
• Be familiar with the available evidence
• Reinforce the current process for consideration of grey indications1:
• Left hand side – IAP can approve (inform NHSE hub pharmacist)
• Right hand side – if IAP support, submit an IFR to NHSE
• Ensure efficacy outcome recording on the database; review and follow up as necessary
• Monitor level of annual reviews conducted in long term use
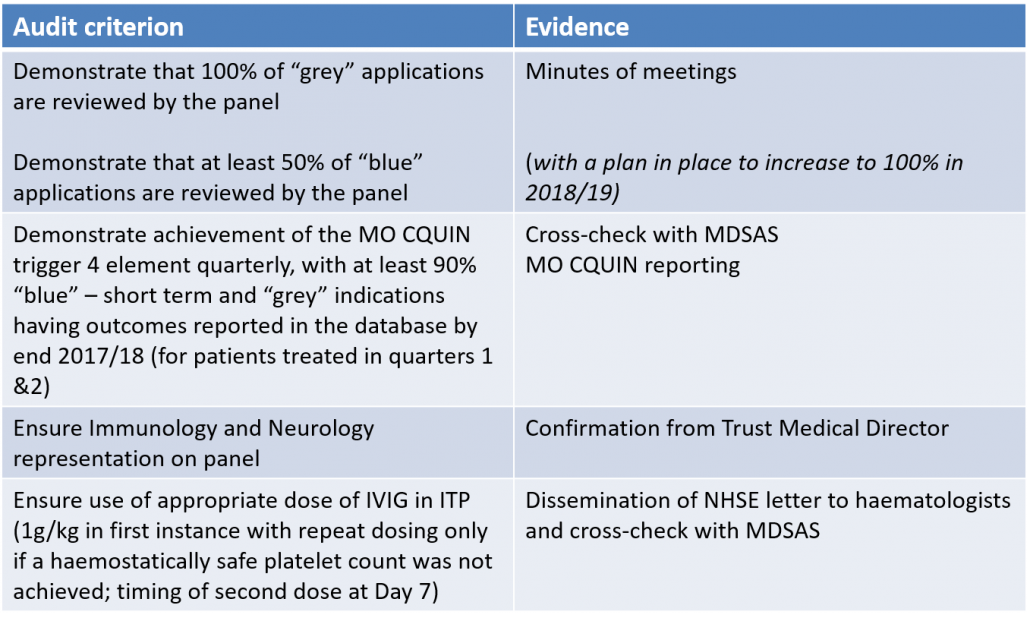
• Audit success of IAP – suggested audit criteria are shown below
• Share best practice amongst trusts